Alkalinity | Sanitary Engineering laboratory
Introduction:
Alkalinity is a parameter that is measured on samples such as drinking water, natural waters, polluted waters, sewage, and industrial wastes. It refers to the buffering capacity of water samples and to their ability to neutralize acid. For municipal sewage or industrial wastes, the amount of alkalinity is important in determining the type of treatment that should be employed.
Objective:
The main objective of this experiment is to determine the alkalinity of a wastewater sample and tap water sample using titration method and comparing the two results.
Theory:
Alkalinity is measured by titration. An acid of known strength is added to a specific volume of a sample. The alkalinity of the sample is reflected by the volume of acid required to bring the sample to a specific pH level. Total alkalinity is measured by measuring the amount of acid (e.g., sulfuric acid) needed to bring the sample to a pH of 4.5.
Three major classes of materials, which are ranked in order of their association with high pH values as follows, (1) hydroxide (OH-), (2) carbonate (CO32-), and (3) bicarbonate (HCO3-), are the major portion of the alkalinity in natural waters:
Most of the natural alkalinity in waters is due to (HCO3-) which is produced by the action of CO2 and H2O on limestone:
CO2 Carbon dioxide originates from bacterial decomposition of organic matter.
Alkalinity is normally divided caustic or phenolphthalein alkalinity above pH 8.3, and total alkalinity above pH 4.5
Total alkalinity = [OH-1] + [CO32-] + [HCO3-]
Alkalinity can be determined using the following equation:
α: the volume (ml) of H2so4 required to change PH to 4.5
N: normality of H2SO4 =0.02
V: volume of sample (ml).
Alkalinity can be expressed in mg/L of equivalent CaCO3 calcium carbonate
Equipment and materials
Equipment:
1- Burette tube.
2- Stand.
3- Magnetic Stirrer plate.
4- Beaker.
5- pH-meter.
6- Graduated cylinder.
Material:
1- Wastewater sample(50ml)
2- Tap water sample (50ml)
3- Sulfuric acid H2SO4 , N=0.02
Procedure:
1- 50 ml of wastewater was prepared and put in a beaker.
2- The initial pH was read using the pH-meter and the initial reading of the burette tube was taken.
3- Titration of the sample was done with sulfuric acid solution with continuous stirring using the magnetic stirrer.
4- The titration was continued until the pH reading was almost 4.5 then the final reading from the burette tube was taken.
5- The same procedure was done for tap water.
Data Collection and Calculations:
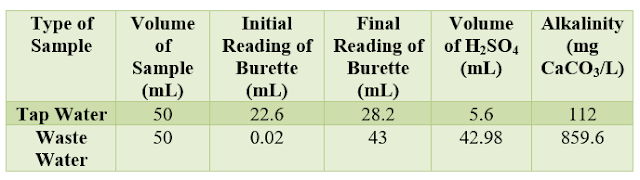
Table 1: Data and Results of alkalinity
Sample Calculation of Waste Water Sample:
Conclusion:
Alkalinity can be defined as: the measure of a solution’s capacity to react with a strong acid (usually this acid is H2SO4 sulfuric acid) to a predetermined pH. The alkalinity of a solution is made of carbonate, bicarbonate, and hydroxides. The higher the alkalinity the more the neutralizing agent needed to counteract it. A treatment plant and its collection system in general operates better with wastewater higher in alkalinity and lower in acidity. It also affects biological processes (such as nitrification) and chemical reactions.
The alkalinity of the waste water at ph= 4.5 is 859.6 mg CaCO3/L. This result is much more than the tap water alkalinity (112 mg CaCO3/L). Moreover, the tap water has a very fast drop in H2SO4 which indicates that its alkalinity is smaller than that of wastewater.
There might be errors in this experiment due to:
- Error in taking the volumes of the samples.
- Error in reading from burette tube.
- Error in reaching the final pH value.
References:
Sanitary lab manual

.jpg)








No comments: